Navigating Investigator-Initiated Trials: A Path to Unbiased Clinical Research

Investigator-Initiated Trials (IITs), also known as investigator-initiated studies or investigator-initiated research, are gaining prominence in the field of clinical research. These trials offer a unique opportunity for unbiased and credible research, free from corporate influences. What Are Investigator-Initiated Trials? Investigator-Initiated Trials are studies conducted by independent researchers or groups of researchers who take on the […]
Selecting the Ideal CRO: 4 Key Factors for Successful Clinical Research Partnerships

When embarking on the path of clinical research, selecting the right Contract Research Organization (CRO) can make all the difference in the success of your trials. The right partner can streamline operations, optimize resource allocation, and ultimately contribute to groundbreaking discoveries. This article outlines some crucial factors to consider when choosing a CRO that aligns […]
Data Integration: The Fundamentals
Within the context of the Clinical Data Management (CDM) framework, data integration involves consolidating diverse sources of clinical research data into an Electronic Data Capture (EDC) system. The ultimate goal is to present the data on the EDC interface. Executing CDM data integration entails tasks such as performing EDC backend programming, validating programming, and maintaining […]
The Top 3 Benefits of Remote Monitoring
Clinical trials play a crucial role in the emergence of new drugs, medical devices, and treatments. Oftentimes, conventional methods of on-site monitoring can cause major delays in the overall progress and time taken to complete these trials. In other cases, traditional methods have proven to be restrictive and fail to comply with regulatory standards. Taking […]
A Step-by-Step Guide to eConsent ICF (Informed Consent Form)
Introduction In the realm of clinical trials and research studies, a contemporary method known as electronic consent, or eConsent, has emerged to revolutionize the way informed consent is obtained from participants. Steering away from the conventional paper-based approach, eConsent leverages digital platforms or software to present participants with essential information and enable them to provide […]
RWE in Market Access & Health Economics: 3 Key Applications & 3 Key Benefits
Real-world evidence (RWE) holds immense significance as a tool for comprehending treatment effectiveness within real-world settings. Notably, RWE offers invaluable insights into the cost-effectiveness of treatments and plays a crucial role in informing decisions pertaining to market access. In this article, we will explore three key applications and three key benefits of RWE in the […]
The Benefits of Real-World Evidence (RWE) for Pharmaceutical Companies
In the realm of healthcare, real-world evidence (RWE) serves as a cornerstone in guiding critical healthcare decisions and assessing the safety and efficacy of treatments. Drawing from various sources, such as patient medical records, claims data, and observational studies, RWE offers invaluable insights into the real-life outcomes of healthcare interventions. By leveraging this wealth of […]
Unleashing Potential for Digital Endpoints: How They Can Revolutionize Clinical Research

Digital endpoints have the potential to revolutionize clinical trials by providing real-time data collection, enhancing patient engagement, improving efficiency, and reducing costs. These new forms of data points are assessed using data captured by a sensor, typically outside of a clinic during daily living activities. The sensor can be worn like a pedometer in a […]
Unlocking the Key Insights: A 4-Part Framework to Characterize Real-World Data (RWD) for Regulatory Compliance
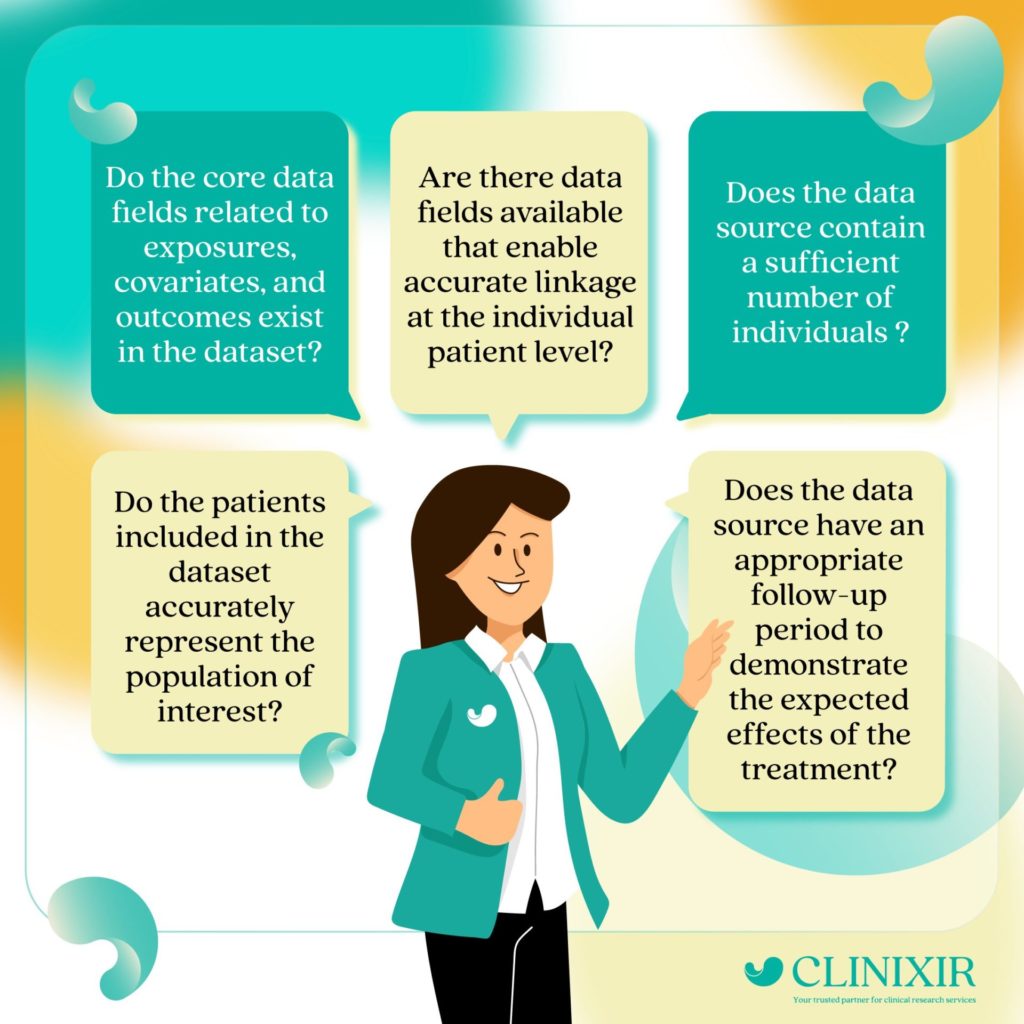
According to Acorn AI, one of the industry’s leading platforms supporting clinical trials, in 2020, approximately 75% of new drug applications (NDA) and Biologic License Applications already incorporate real-world data. Since then, there has been a notable increase in the utilization of RWD. However, like with any pioneering advancement, there exists challenges between the potential […]
The Importance of Quality Assurance and Control in Clinical Research

Clinical research stands at the forefront of medical advancements and the evolution of effective therapies and treatments. To establish unwavering trust in the results, studies must be conducted with uncompromising attention to detail. In this context, quality assurance and control emerge as indispensable pillars, safeguarding the delivery of the highest quality and most reliable outcomes. […]


